Strep-tag® - Leading affinity chromatography system
From purification to analytical applications
Our proprietary Strep-tag® technology exploits one of the strongest non-covalent interactions in nature: the interaction of biotin and streptavidin. The system is based on the highly selective and easily controllable interaction between the synthetic Strep-tag®II peptide and the specially engineered streptavidin, called Strep-Tactin®, which is one of the most stable proteins known. The Strep-tag®II binds specifically to the engineered streptavidins, Strep-Tactin® and Strep-Tactin®XT, by occupying the binding pocket of the natural ligand biotin. Hence, the interaction is easily reversible by excessive addition of the competitor.
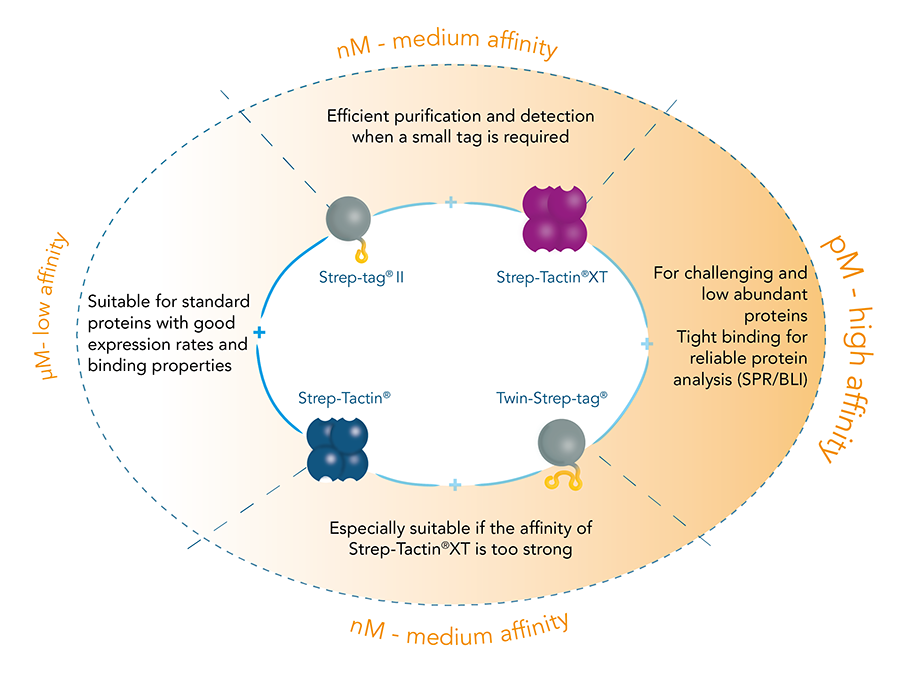
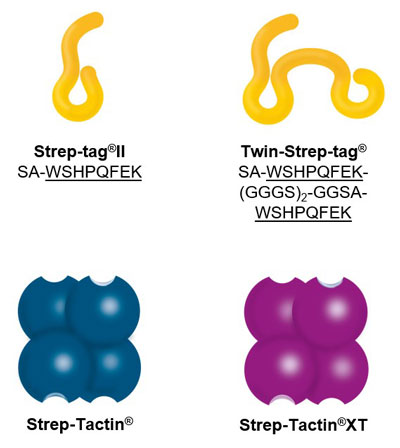
Two affinity tags – two streptavidin derivatives: The Strep-tag®II consists of eight amino acids (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys), whereas the Twin-Strep-tag® includes this motif two times in series connected by a linker and is accordingly composed of 28 amino acids. Both exhibit intrinsic, although unequal, affinity towards the streptavidin derivative Strep-Tactin® and its successor Strep-Tactin®XT: The binding affinity of Strep-tag®II to Strep-Tactin® (1µM) is nearly 100 times higher than to streptavidin. A further improvement was achieved by the development of Strep-Tactin®XT, which shares a nM affinity with the Strep-tag®II and a pM affinity with the Twin-Strep-tag®.
The Strep-tag® protein purification system comprises two affinity tags, the 8 aa Strep-tag®II and its tandem version Twin-Strep-tag®. Both versions can bind to Strep-Tactin® and its high affinity variant Strep-Tactin®XT. Thereby the two tags differ in the affinities with which they bind to Strep-Tactin® and Strep-Tactin®XT. Depending on the application and properties of the protein of interest one can combine the different tags and Strep-Tactin® variants according to the required affinity.
As a result of the differences in binding strength among the possible tag-ligand combinations, the Strep-tag® system has become established as a universal tool for isolation of proteins and cells.
- Fused to recombinant proteins, the Strep-tag® enables efficient one-step purification on immobilized Strep-Tactin®.
- When fused to antibody-derived Fab fragments or nanobodies, Twin-Strep-tag® binds to multimerized Strep-Tactin® allowing capturing and releasing of target cells based on their surface-marker or antigen-specificity.
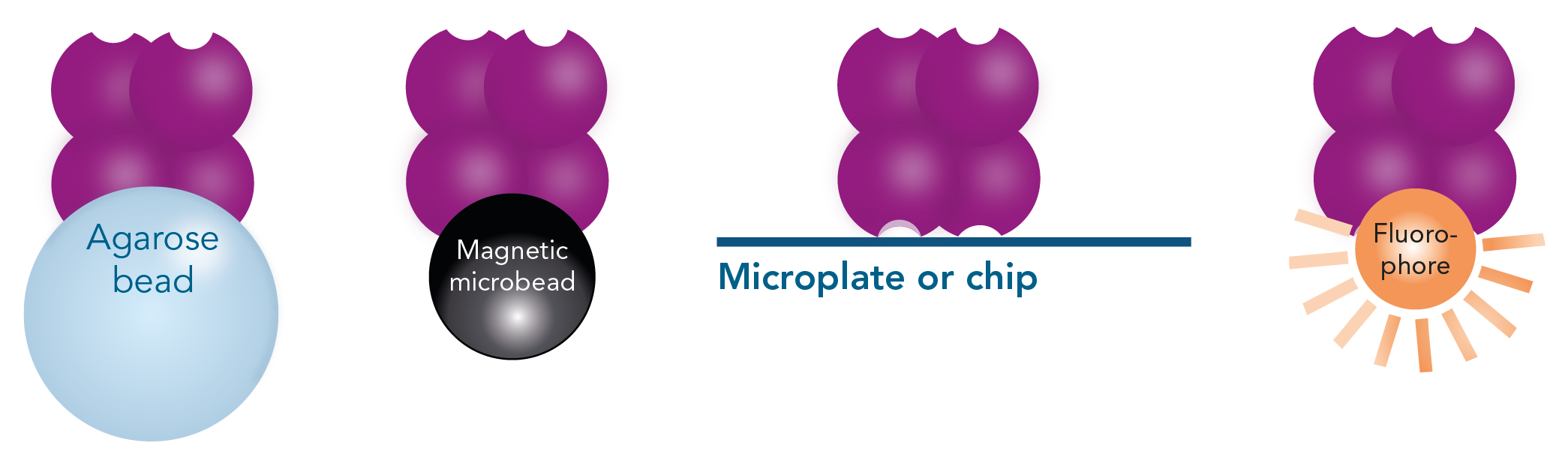
- Strep-Tactin® (or Strep-Tactin®XT), on its turn, can be conjugated to microplates, fluorophores or chips, allowing a wide range of analytical applications after isolation of target material, such as detection, immobilization and interaction studies.
Key features of the Strep-tag® system are:
- Reversibility
- Full functionality of target material after isolation
- High purity
- Universal applicability for various analytic applications
- Simple purification procedures