Scalable protein purification with MagStrep® Strep-Tactin®XT beads
Protein purification
Affinity chromatography is a pivotal technique in protein purification, leveraging specific interactions between proteins and ligands for precise isolation. Among the various methods, pull-down assays stand out for studying protein-protein interactions, effectively capturing target proteins using a ligand attached to a solid support. For large-scale applications, upscaling techniques ensure efficient and consistent purification, accommodating industrial demands. In the context of high-throughput purification, automated systems and optimized protocols enable the processing of numerous samples simultaneously, significantly accelerating research and development processes. Additionally, magnetic beads purification offers a versatile and rapid approach, ideal for both small and large-scale applications. Discover our protein purification formats , including pre-packed gravity flow columns, FPLC columns, resins, and magnetic beads suited to different needs.
Protein purification with magnetic beads allows for efficient isolation of proteins especially from small sample volumes or even viscous material. MagStrep® Strep-Tactin®XT beads are perfectly suitable for batch purification of proteins, since they are easily separated from the supernatant. The complete removal of sample material during the magnetic bead purification process in combination with the high specificity of these beads results into an exceptionally high purity. Additionally, magnetic bead-based purification can be easily scaled up or down to accommodate different sample sizes. A quick protocol allows an easy transfer to automated systems, which is ideal for high-throughput screenings.
Efficiency

Fig. 1. GFP-Twin-Strep-tag samples were incubated with magnetic beads for different periods of time. An incubation time of only 10 minutes is sufficient to purify almost the entire amount of protein.
Magnetic bead-based purification is rapid and efficient. The magnetic properties of the beads enable easy and rapid separation of the beads from the supernatant using a magnet. This reduces processing time and allows for quick purification of proteins.
Depending on your experimental conditions, it's possible to optimize the purification protocol and increase protein yield by adjusting specific parameters. The following tips and data will help you optimize magnetic bead protein purification by adjusting bead volume, incubation time, and protein concentration.
Scalability

Fig. 2. Scalability of the protein purification with MagStrep® Strep-Tactin®XT beads was tested by purifing αCD45 nanobody fused to a Twin- Strep-tag® from different samples sizes. Scaling up the purification results in an increasing amount of isolated protein while maintaining the yield per ml of lysate.
Automation

Fig. 3. MagStrep-Tactin®XT beads were tested using a Tecan Fluent®. Consistently high recovery and purity was achieved across all samples.
Magnetic bead-based purification can be easily automated using robotic systems. Automated protein purification systems are fast and deliver reproducible results, making them ideal for high-throughput applications such as drug discovery and screening.
Efficiency and purity in magnetic bead-based protein purification
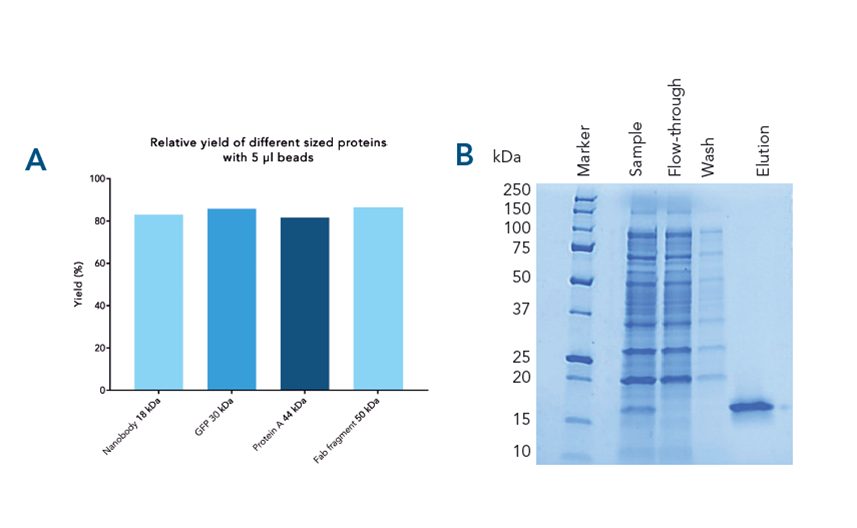
Specific capture, high yield and target protein purity . Different proteins were purified from spiked E. coli lysate with high recovery (> 82%) (A). Specific capture and purity is shown for αCD45 nanobody fused with the Twin-Strep-tag® as an example (B).

Time-saving protein purification. GFP-Twin-Strep-tag samples were incubated with magnetic beads for different periods of time. An incubation time of only 10 minutes is sufficient to purify almost the entire amount of protein.
Easy scalable magnetic bead-based protein purification with option for re-use
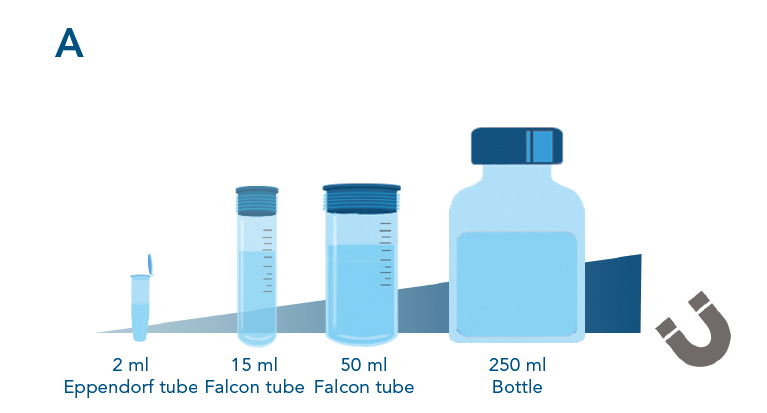
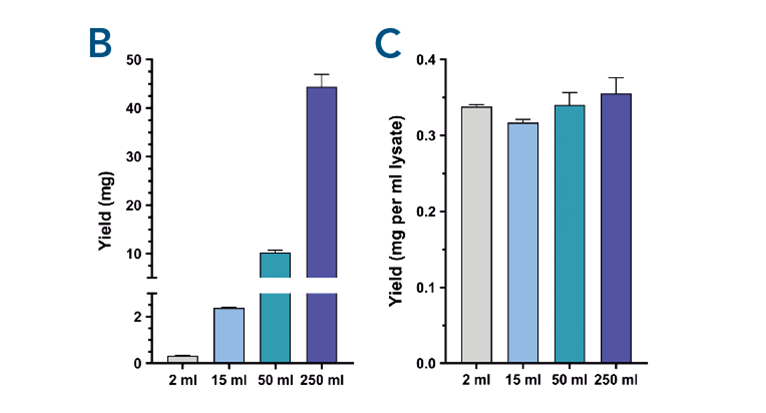
Easy scalability of the purification process. Scalability of the protein purification with MagStrep® Strep-Tactin®XT beads was tested by purifing αCD45 nanobody fused to a Twin- Strep-tag® from different samples sizes. Separation occurred with a SepMag® A200ml biomagnetic separator (A). Scaling up the purification results in an increasing amount of isolated protein (B) while maintaining the yield per ml of lysate (C).
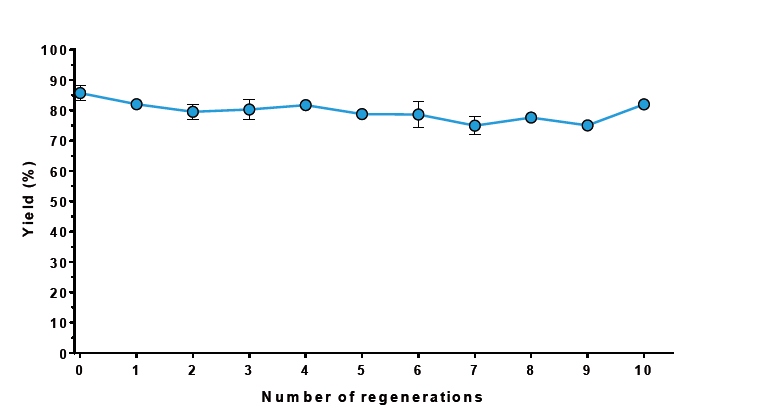
Fast and easy regeneration. After each purification, MagStrep® Strep-Tactin®XT beads were regenerated with 0.1 M NaOH for 2 min. Protein binding capacity remained stable for at least 10 regeneration cycles, saving costs especially in large-scale applications.
High-throughput screening and automation with MagStrep® Strep-Tactin®XT beads
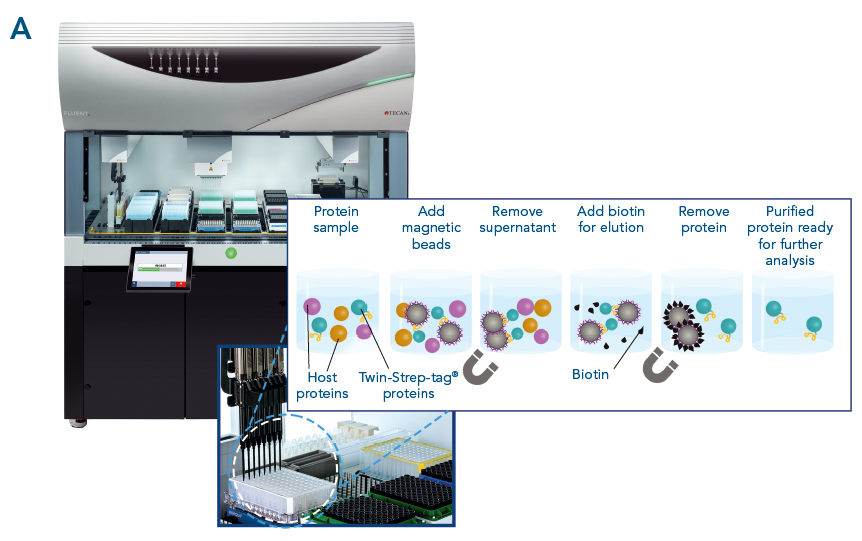
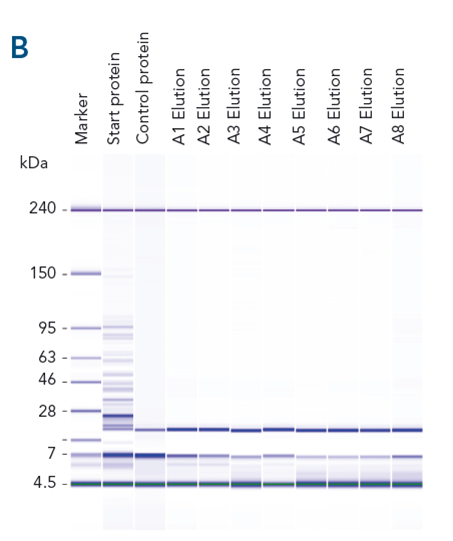
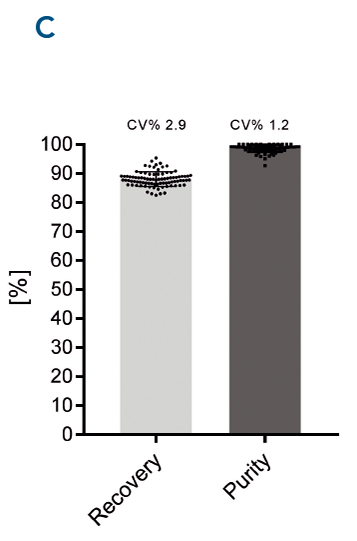
Automated protein purification with high recovery and purity. Automation and parallel protein purification with MagStrep® MagStrep-Tactin®XT beads were tested using a Tecan Fluent® (A). αCD45 nanobody fused to a Twin-Strep-tag® was purified in parallel from 88 samples. Purity was analyzed using Bioanalyzer on a Protein 230 Chip (B). Consistently high recovery and purity was achieved across all samples (C).
References for possible applications using magnetic beads
Small-scale purification:
"GPCR Solubilization and Quality Control. Expression, Purification, and Structural Biology of Membrane Proteins." (Miljus, T., et al. (2020)..)
Solubilization of G protein-coupled receptors (GPCRs) is challenging due to their hydrophobic nature and instability in aqueous solutions. Miljus et al., outlines methods for extracting GPCRs using detergents and subsequently assessing the quality and functionality of the solubilized proteins. For effective small-scale purifications of GPCRs, Miljus et al. describe a straightforward procedure using Strep-Tactin®XT coated magnetic beads combined with the Twin-Strep-tag®.
Affinity-purification coupled with mass spectroscopy (AP/MS):
"A comprehensive proteomics analysis of JC virus Agnoprotein-interacting proteins: Agnoprotein primarily targets the host proteins with coiled-coil motifs." (Saribas, A. S., et al. (2020)..)
The paper focuses on the JC virus (JCV) Agnoprotein and its role in the virus's replication cycle. Agnoprotein, identified as critical for JCV replication, interacts with 501 host cellular proteins, which was explored using the Strep-tag® affinity purification system and mass spectroscopy (AP/MS). These interactions span across multiple cellular networks involved in protein synthesis, degradation, and cellular transport. The interaction landscape extends to organelles like mitochondria, the nucleus, and the ER-Golgi network. Saribas et al. preferred the Strep-tag over the Flag-tag and His-tag due to its high specificity and affinity, mild purification conditions, biological inertness and stability, and high purity which is crucial in highly sensitive applications like proteomics.
Automation:
"A SARS-CoV-2 protein interaction map reveals targets for drug repurposing." (Gordon, D. E., et al. (2020).)
Gordon et al., identified the human proteins that associate with viral proteins of SARS-CoV-2 using an affinity-purification mass spectrometry (AP-MS) approach. In this regard, they expressed Twin-Strep-tagged versions of SARS-CoV-2 proteins in human cells, which they could verify by anti-Strep western blot. Next, Gordon and colleagues purified the expressed Twin-Strep-tagged viral proteins together with associated human proteins using the predecessor of the MagStrep® Strep-Tactin®XT beads in combination with the KingFisher Flex Purification System, an automated extraction instrument. This high-throughput approach identified 332 high-confidence human proteins interacting with virus proteins, essential for drug repurposing efforts against COVID-19.