How to circumvent common problems associated with the His-tag system?
The His-tag (6xHis-tag, His6-tag or polyhistine-tag) is a popular affinity tag for protein purification since its small size ensures a low impact on protein structure. While the His-tag system is popular for its high yield and low costs, the time and effort it takes to establish successful His-tag purification is often neglected.
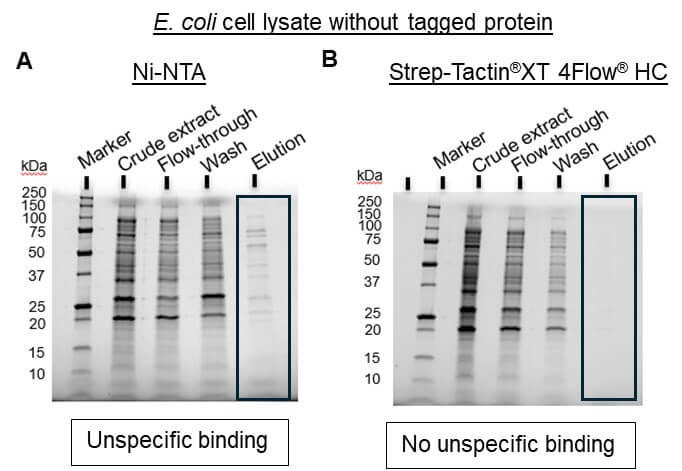
- Low target purity due to unspecific binding of host proteins (Figure 1A)
- Sample or buffer components interfere with protein binding
- Protein precipitation after purification due to incompatible buffer components
- Culture media for mammalian cells interfere with protein binding or cause Ni2+ leakage
- Required pH for target protein causes elution from Ni-NTA agarose
- Limited applicability in protein analysis due to low tag-ligand affinity and specificity
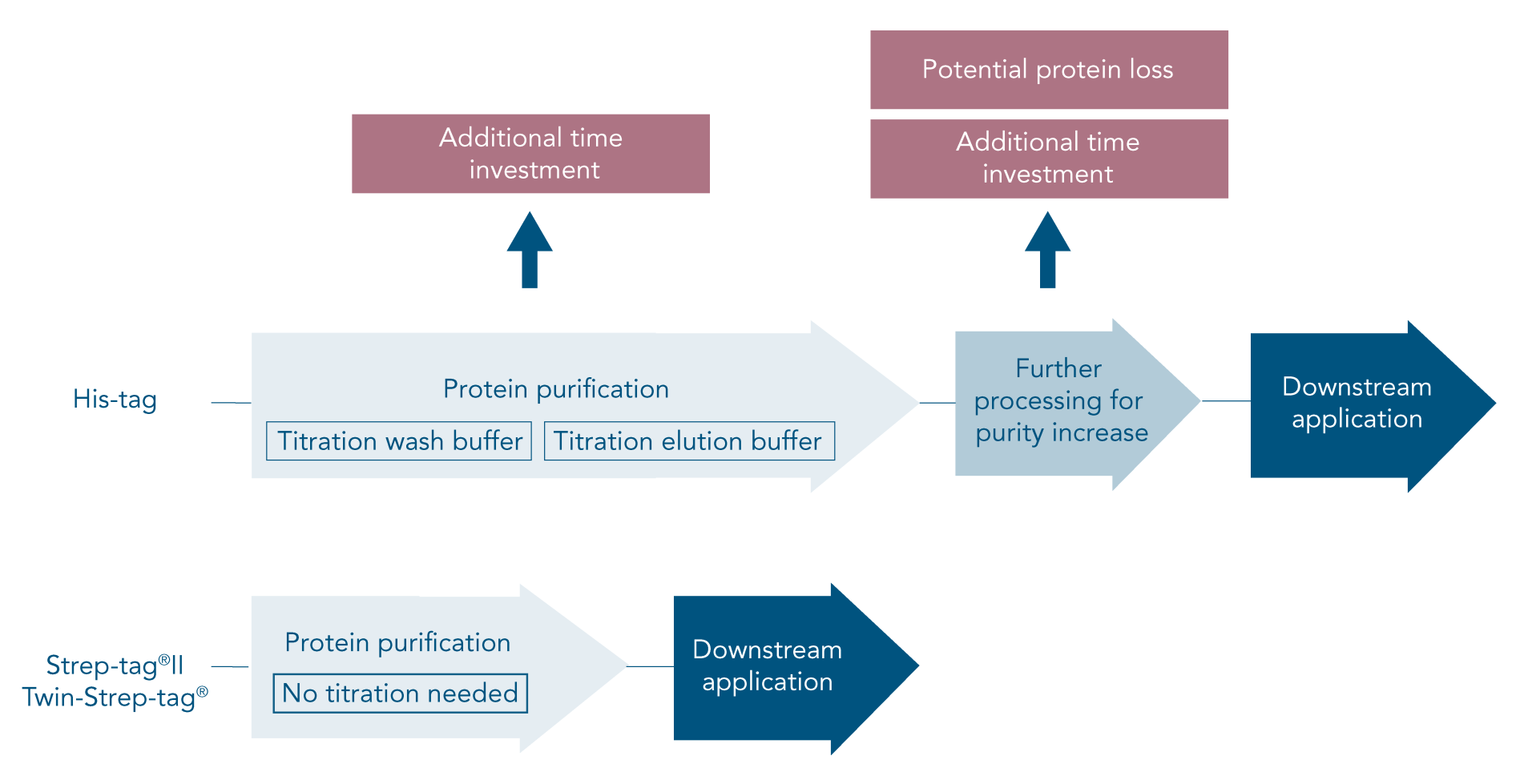
To avoid or address these problems, careful planning of the procedure and optimization of the His-tag purification protocol is required. In the end, a His-tag might still not achieve satisfactory results or might not be suitable at all for a certain type of protein. In this case, a different affinity tag should be considered.
The Strep-tag® system is the ideal alternative to His-tag since all common problems are avoided. Due to the specific tag-ligand interaction, protein isolation using this system leads to highly pure protein without further optimization or processing steps (Figure 1B).
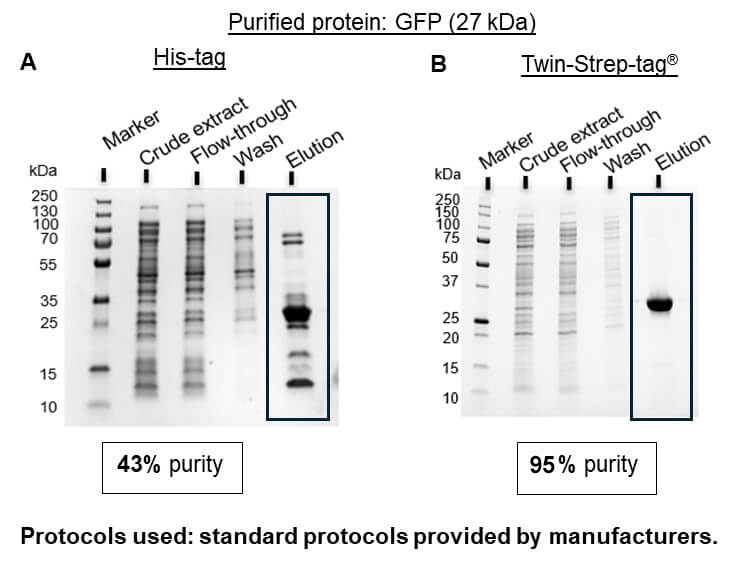
Figure 1: GFP fused to either a 6xHis-tag (A) or to a Twin-Strep-tag® (B) was isolated from E. coli lysates using standard protocols provided by the manufacturers for the used resins (Ni-NTA from Thermo Fisher Scientific for His-tag and Strep-Tactin®XT 4Flow® high capacity from IBA Lifesciences for Twin-Strep-tag®)
Watch our free on-demand about common problems during His-tag purification and read our white paper comparing His-tag and Strep-tag® protein purification systems.