Cost-saving protein research due to simple & efficient regeneration of Strep-Tactin® and Strep-Tactin®XT
The ability to reuse purification or immobilization products saves both time and costs by reducing the need for frequent replacements. This benefit extends to a range of applications, including affinity chromatography, magnetic batch purification, and SPR/BLI. To allow for an effective re-use without loss of binding capacity, a regeneration procedure that is efficient but not detrimental to the binding ligand and its supporting material is required. Due to their binding properties as well as their high stability, Strep-Tactin® and Strep-Tactin®XT coupled to magnetic or non-magnetic agarose beads or to biosensor chips are perfectly suitable for multiple regeneration cycles and even clean-in-place (CIP) procedures.
Regeneration of affinity chromatography resins
Strep-Tactin® and Strep-Tactin®XT affinity chromatography resins are easily regenerated with 100 mM sodium hydroxide (NaOH). It is a simple procedure in which the regeneration buffer is directly applied after the final protein elution step. After washing the column, it can immediately be used for further purification steps or stored for later usage (Fig. 1). Since the ligands (Strep-Tactin® & Strep-Tactin®XT) are stably coupled to the agarose beads, resins can be regenerated 50 times* without losing its binding capacity (Fig. 2). For Strep-Tactin®XT resins, IBA Lifesciences also offers a ready-to-use regeneration buffer (Buffer XT-R) containing 3 M MgCl2 that can be used as effectively as 100 mM NaOH (Fig. 2 right).
*may vary depending on protein properties and required buffer components
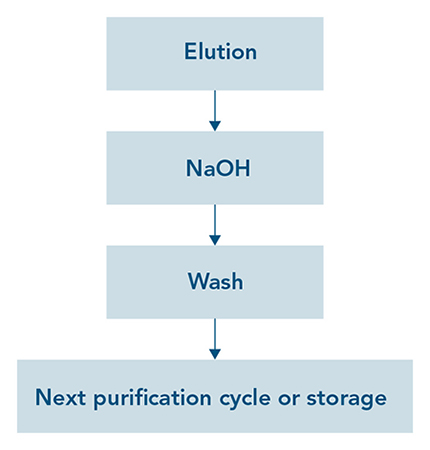
Figure 1: Quick & easy regeneration principle for Strep-Tactin® & Strep-Tactin®XT resins. After eluting the bound protein, 100 mM NaOH can be applied for regeneration. After washing with Buffer W, the resin is ready for another purification cycle or for storage.
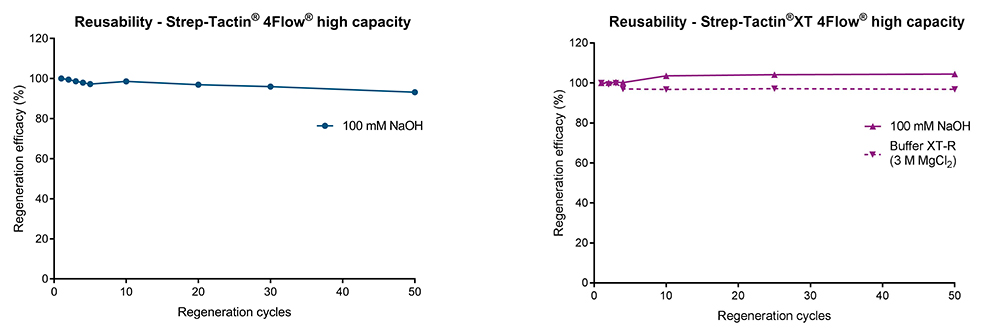
Figure 2: (left) Strep-Tactin® and (right) Strep-Tactin®XT affinity chromatography resins can be regenerated 50 times with 100 mM NaOH without loss in binding capacity. For Strep-Tactin®XT, a ready-to-use regeneration buffer (Buffer XT-R) containing 3 M MgCl2 is offered for convenient use.
Clean-in-place (CIP)
In bioprocessing applications, it is not only relevant that an affinity chromatography resin can be re-used multiple times to reduce costs, but also that it can be cleaned efficiently to prevent column fouling. Impurities need to be removed to avoid sample contamination as well as a decline in column performance. A standard cleaning agent in bioproduction is NaOH. A sufficiently high concentration of NaOH removes i.e. proteins, lipids, nucleic acids as well as bacteria. While highly stable at 100 mM NaOH (Fig. 2) Strep-Tactin® and Strep-Tactin®XT resins can also be exposed to 500 mM NaOH at least 10 times without affecting the resin performance (Fig. 3). Even after 50 cycles, only a marginal decline in binding capacity is observed, demonstrating the suitability of Strep-Tactin® and Strep-Tactin®XT resin for CIP procedures.
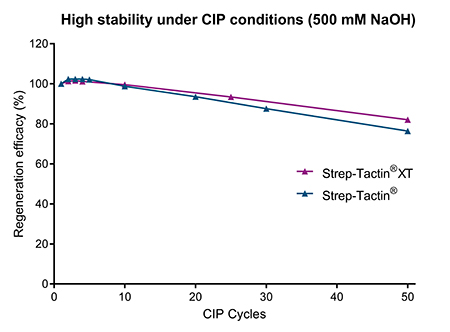
Figure 3: Strep-Tactin® as well as Strep-Tactin®XT resins can be exposed to 500 mM NaOH during several CIP cycles without a significant loss in binding capacity.
Regeneration of magnetic beads
An alternative for affinity chromatography-based protein purification is batch purification using magnetic beads. Magnetic beads are especially popular for small-scale and high throughput applications. However, upscaling is also possible, which makes the option for regeneration key for a cost-effective usage. MagStrep® Strep-Tactin®XT beads are agarose-coated magnetic beads that can be re-used at least 10 times if regenerated with 100 mM NaOH after every purification (Fig. 4). Similar as for affinity chromatography resins, the regeneration procedure is simple and straightforward, requiring only two basic steps: incubation with NaOH and subsequent wash.
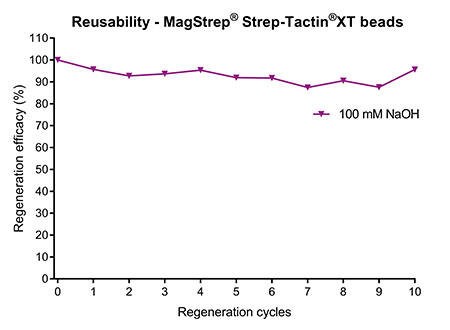
Figure 4: MagStrep® Strep-Tactin®XT beads were regenerated with 100 mM NaOH after protein purification. The binding capacity remained stable over at least 10 regeneration cycles.
Regeneration of SPR chips
The properties of Strep-Tactin®XT not only enable efficient regeneration in protein purification approaches, but also in analytical applications such as SPR or BLI. For kinetic measurements, Strep-Tactin®XT is immobilized on biosensors. After capturing a strep-tagged ligand and measuring the binding kinetics of an analyte, addition of 3 M guanidine hydrochloric acid (GuHCl) efficiently removes all ligand-analyte complexes. After GuHCl is washed away with an appropriate running buffer, another sample can be analyzed or the biosensor stored until further use (Fig. 5 left). With this simple procedure, Strep-Tactin®XT coated chips can be re-used at least 30 times without any negative effects on the capture level of the ligand (Fig. 5 right). In addition to saving costs, this option of easy regeneration is highly convenient for measuring several samples consecutively, since Strep-Tactin®XT remains stably bound to the biosensor after every cycle.
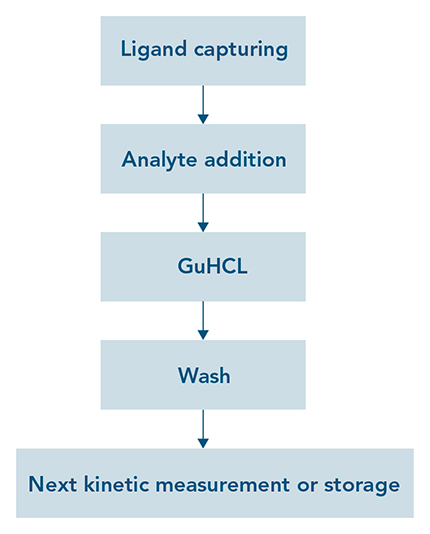
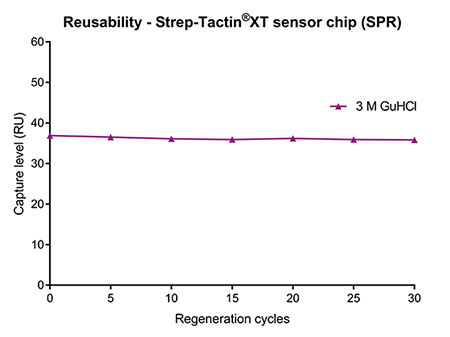
Figure 5: (left) Strep-Tactin®XT coated on biosensors enables a simple & straightforward regeneration procedure in analytical applications such as SPR and BLI. (right) A Strep-Tactin®XT sensor chip was regenerated with 3 M GuHCl after each measurement. The capture level of a Twin-Strep-tagged protein ligand remained stable over at least 30 regeneration cycles.

