Protein purification product formats
Protein purification via affinity chromatography is based on the binding affinities between different molecules. During purification, target protein tagged with a short peptide binds to the ligand, which is usually coupled to resin, while unwanted proteins without tag do not bind and can be washed away.
The resin can consist of agarose or magnetic beads. Both come in suspension form. Alternatively, agarose beads can also be prepacked in FPLC, gravity or spin columns. Which format works best for your application depends either on the yield you require or on the initial sample volume.
Choosing the product format based on...
Yield

Sample volume
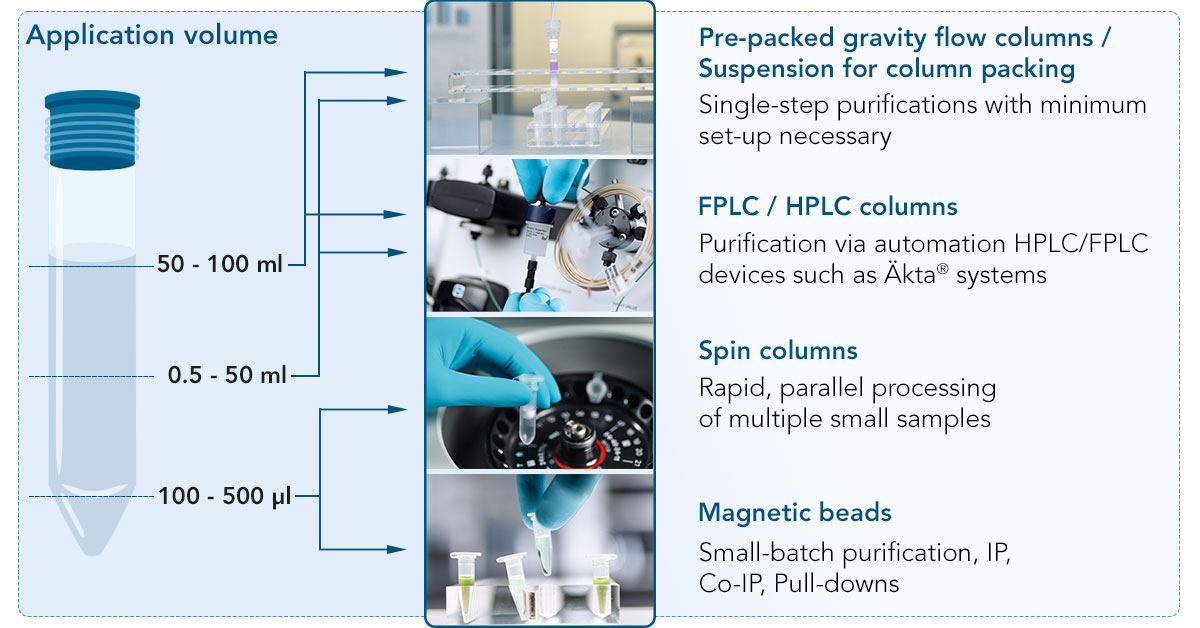
Product formats in details
FPLC/HPLC columns
Large sample volumes can be purified using columns in automated FPLC/HPLC devices such as Äkta®. FPLC columns come with different resin volumes and can be connected in series to increase the binding capacity. They attach to the device via the standard 10-32 connectors.
- Broad pH compatibility
- Excellent pressure stability
- Outstanding target purity
- 1 & 5 ml column bed volume available
- Quick application of large sample volumes
- Fully automated protein purification
- Time saving: No waiting between the purification steps, since the device applies the correct volumes of buffer and sample autonomously.
- Recording of the complete affinity chromatography protocol, which provides information about the purification quality
- Reproducible purification results due to the program settings that are set up beforehand and not dependent on the user


Gravity flow columns
Gravity flow columns are ideal for protein purifications with a medium yield. The resin suspensions allow you to pack your own columns according to your need, but you can also choose from other formats, like pre-packed columns.
- Optimized for column affinity chromatography
- Variety of different product sizes and matrices
Prepacked columns
Suspension
Magnetic beads
Magnetic beads can be used for batch purifications in tubes or 96-well plates to purify proteins in pull-downs, IPs and Co-IPs. The beads can be easily fixed to the reaction tube with a magnet, so you can skip centrifugation without having to worry about losing your sample during pipetting steps.
- No centrifugation
- Separation via magnetic separator
- 2 ml and 10 ml volume available

Spin columns
If you need to process multiple small samples in parallel, you can use spin columns with the resin of your choice. They allow you to do a batch purification of a different protein in every tube with a simple setup.
- Compatible with conventional 1.5 ml or 2.0 ml reaction tubes
- Buy a ready-to-use kit or fill empty columns with resin of your choice

Resin reuse
Strep-Tactin® and Strep-Tactin®XT resins for protein purification can be regenerated and reused >10 times* without loss in performance. Resin activation can easily be checked with HABA. The yellow HABA solution turns red (Strep-Tactin®) or orange (Strep-Tactin®XT) upon binding to the engineered biotin binding pockets of Strep-Tactin® and Strep-Tactin®XT clearly indicating that the resin is fully regenerated. Afterwards, HABA can be removed by washing with 100 mM NaOH. Once the red color has disappeared and NaOH was removed from the column using Buffer W, the column can be reused. If the biotin binding pocket is blocked or damaged no color shift occurs and the resin cannot be reused.
*under ideal conditions >50 regeneration cyles are possible

Resin specifications
|
Strep-Tactin® |
Strep-Tactin®XT |
||||
|---|---|---|---|---|---|
| Matrix | Superflow® | Sepharose® | MacroPrep® | 4Flow® | MagStrep Strep-Tactin®XT beads |
| (only available as 20 ml slurry) | |||||
| Binding capacity*/ Dynamic Binding capacity** |
classic: 3 mg/ml resin* | classic: 3 mg/ml resin* | classic: 3 mg/ml resin* | classic: 5 mg/ml resin** | classic: 25.5 µg/µl resin* |
| high capacity: 7.0 mg/ml resin** | high capacity: 14 mg/ml resin** | ||||
| Bead structure | 6% agarose, crosslinked | 4% agarose, crosslinked | polymethacrylate | 4% agarose, highly crosslinked | magnetic core covered with 6% agarose, crosslinked |
| Bead size | 60-160 µm, spherical | 45-165 µm | 50 µm | 50-150 µm, spherical | 30 μm (average), spherical |
| Exclusion limit | 6 x 106 Da | ~3 x 107 Da | 1 x 106 Da | 3 x 107 Da | not specified |
| Recommended flow rate | 0.5-1 ml/min | gravity flow | 0.5-1 ml/min | 0.5-1 ml/min | batch purification |
| pH range for protein binding | 7-8 | 7-8 | 7-8 | 4-10 | 6-10 |
| Max pressure | 9.6 bar | gravity flow | 70 bar | 3.5 bar | batch purification |
| Storage | 4 °C, do not freeze | 4 °C, do not freeze | 4 °C, do not freeze | 4 °C, do not freeze | 4 °C, do not freeze |
| Shipment | RT | RT | RT | RT | RT |
| Eluent | desthiobiotin |
desthiobiotin |
desthiobiotin |
biotin | biotin |
| Regeneration buffer | Buffer R | Buffer R | Buffer R | Buffer XT-R 0.1 M NaOH |
0.1 M NaOH |
| Features and recommendations |
|
|
|
|
|
|
* Max binding capacity for a Strep-tag® protein (30kDa). ** Dynamic binding capacity (DBC) was determined with mCherry-Twin-Strep-tag (30 kDa) under realistic operation conditions and shows the amount of protein which is bound until 10% of the protein is found in the flow through. |
|||||
Resin specifications
| Ligand | Strep-Tactin® | Strep-Tactin®XT | ||
|---|---|---|---|---|
| Matrix | 4Flow® high capacity | 4Flow® | 4Flow® high capacity | MagStrep® Strep-Tactin®XT beads |
| Binding capacity | 20 mg/ml resin* | 11 mg/ml resin* | 31 mg/ml resin* | 42.5 mg/ml beads* |
| Bead structure | 4% agarose, highly crosslinked | 4% agarose, highly crosslinked | 4% agarose, highly crosslinked | magnetic core covered with 6% agarose, crosslinked |
| Bead size | 50-150 µm, spherical | 50-150 µm, spherical | 50-150 µm, spherical | 30 μm (average), spherical |
| Exclusion limit | 3 x 107 Da | 3 x 107 Da | 3 x 107 Da | not specified |
| Recommended technique | Gravity flow, FPLC | Gravity flow, FPLC, centrifugation | Gravity flow, FPLC, centrifugation | Batch purification |
| pH range for protein binding | 7-8 | 4-10 | 4-10 | 6-10 |
| Max pressure | 3.5 bar | 3.5 bar | 3.5 bar | |
| Storage | 2-8 °C, do not freeze | 2-8 °C, do not freeze | 2-8 °C, do not freeze | 2-8 °C, do not freeze |
| Shipping | RT | RT | RT | RT |
| Eluent | desthiobiotin | biotin | biotin | biotin |
| Regeneration buffer | 100 mM NaOH | Buffer XT-R 100 mM NaOH |
Buffer XT-R 100 mM NaOH |
100 mM NaOH |
| Activity Test | Buffer R (HABA) | Buffer R (HABA) | Buffer R (HABA) | |
| Features and recommendations |
|
|
|
|
|
*determined with a 50 kDa Twin-Strep-tag® fusion protein |
||||
Application example
Cell culture supernatant of MEXi-293E cells (24 ml) was spiked with 240 µg of a large protein (130 kDa) C-terminally tagged with the Twin-Strep-tag®. Protein purification was carried out with a 0.2 ml Strep-Tactin®XT 4Flow® high capacity gravity flow column. After sample application the column was washed with 1x Buffer W. The Twin-Strep-tag® protein was eluted by three elution steps with 0.7, 1.5 and 0.8 CV 1x Buffer BXT. Purification results were analyzed by SDS-PAGE (A) and western blot (B). Both show a molecular weight marker (M*, Precision Plus Protein™ Unstained Protein Standards, Strep-tagged recombinant (BioRad), M** PageRuler™ Plus Prestained Protein Ladder (Thermo Fisher Scientific)), the spiked cell supernatant (S), flow through (FT), the 1st and 5th wash fraction (W1 and W5, respectively) and the elution fractions (E1-E3). Strep-Tactin® HRP was applied (1:4000) for detection via western blot.