Surface Plasmon Resonance (SPR)
Surface plasmon resonance (SPR) is an optical biosensor application for direct, real-time, and label-free measurements. It allows the determination of protein concentrations as well as the analysis of affinity and kinetics of a specific protein to other proteins, DNA, RNA, or cells. Therefore, SPR is an innovative tool for biomolecular interaction studies and plays an essential role in biotherapeutic drug discovery and development. The principle of SPR is an optical measurement that detects binding of a biomolecule in solution (analyte) to a biomolecule that is immobilized on a gold-layered surface of a biosensor chip (ligand). Thereby, changes in the refractive index are determined which are proportional to the concentration of bound analyte at the surface of the biosensor chip.
A crucial step in SPR analysis is the immobilization of the ligand to the surface of the biosensor chip. The immobilization of the ligand can take place via direct covalent coupling to the surface of the chip or by capture with the help of a pre-coated capture molecule. However, the direct covalent immobilization of the ligand can change its biological activity or result in an undirected immobilization with reduced accessibility of the binding sites. The transient immobilization of tagged ligands via affinity capturing molecules overcomes these drawbacks.
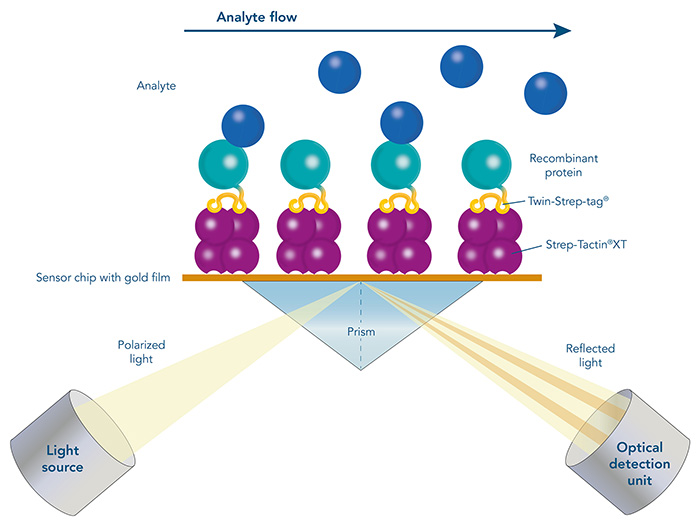
Surface plasmon resonance (SPR) with Strep-Tactin®XT coated sensor chips
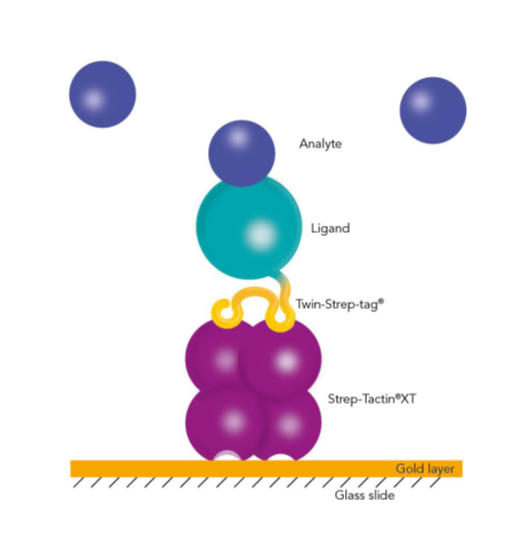
Capturing Twin-Strep-tag® proteins with BiacoreTM
Nevertheless, tags used for such applications must bind efficiently to the capturing molecule with an affinity that is higher than that between analyte and ligand. His-tagged ligands that are immobilized on an NTA-surface with a KD in the nanomolar range can be used for measuring weaker interactions, but they fail in the measurement of high-affinity interactions with slow dissociation rates. In contrast, Strep-Tactin®XT forms an exceptionally strong complex with the Twin-Strep-tag®. An affinity in the low picomolar range allows measurements of long dissociation times and slow off-rates.
Thus, the combination of these two components of the Strep-tag® technology enables a directed, high-affinity immobilization that does not influence the activity of the ligand or require any modifications. Due to the high-affinity interaction and its high specificity, non-specific binding of host cell proteins is avoided, and ligands can be captured efficiently and directly from culture media. Moreover, Strep-Tactin®XT is compatible with many substances and the biosensors can be easily regenerated. The Twin-Strep-tag® Capture Kit provides all necessary products for the preparation of Strep-Tactin®XT-coated biosensor chips and the following measurements.
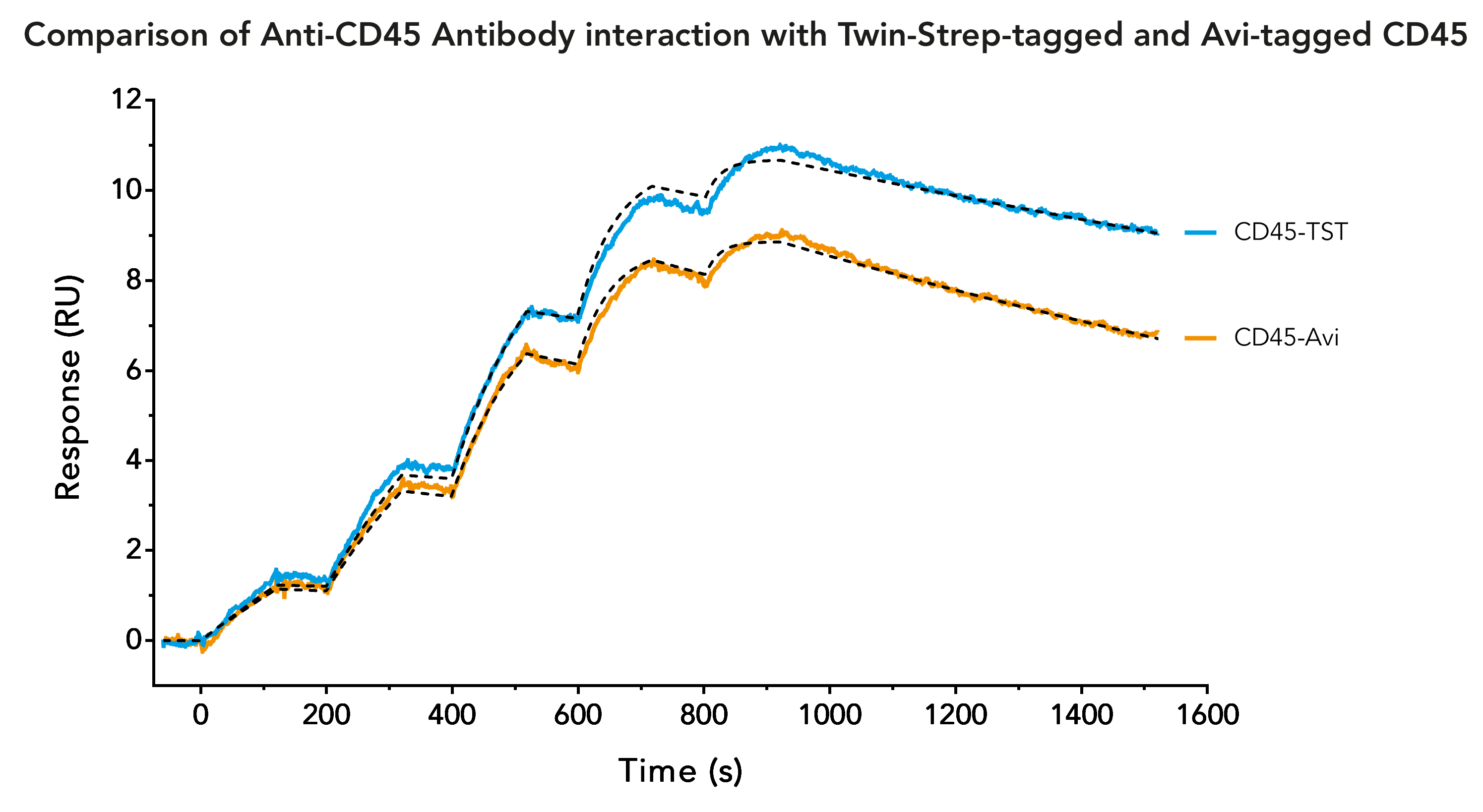
Twin-Strep-tag® - a reliable and time saving alternative to Avi-tag in Surface Plasmon Resonance (SPR) analysis
Surface plasmon resonance (SPR) is a powerful method to study biomolecular interactions. Due to its high accuracy and ability to measure samples in high throughput, it plays a major role in research and development of drugs.
A common tag which is frequently used to immobilize ligands on SPR biosensor chips is the Avi-tag. It is characterized by its high binding affinity and specificity for streptavidin. The combination of Avi-tag and streptavidin allows long-term measurements of interactions with high affinity and slow dissociation rates.
Successful SPR measurements require a sufficient amount of highly pure ligand. Although its binding to streptavidin is very strong, the Avi-tag is not an ideal tag for initial purification of a chosen ligand. The available resins for purifying Avi-tag fusion proteins are expensive and only yield a low amount of protein. The solution for this is the use of a second, more suitable, tag for initial ligand purification. Due to its high purity in protein purification, as well as higher yield and lower costs, the Twin-Strep-tag® is often considered as second tag. Therefore, a frequently asked question is, if the Twin-Strep-tag® and the Avi-tag can be used on the same protein.
The answer is: Twin-Strep-tag® and Avi-tag should not be used on the same protein! The biotinylation of Avi-tag can interfere with the binding of Twin-Strep-tag®, since the binding partner of Twin-Strep-tag® is a streptavidin mutant called Strep-Tactin®XT. Strep-Tactin®XT still has an affinity to biotin and biotinylated proteins, which can affect SPR measurements. Conversely, Twin-Strep-tag® still has a weak affinity to streptavidin, which might influence SPR measurements as well.
The solution to the incompatibility of Avi-tag and Twin-Strep-tag® is quite simple. Avi-tag is not needed for SPR measurements, since Twin-Strep-tag® can be used just as effectively for protein immobilization. In addition to the suitability for the whole procedure from purification to immobilization, using Twin-Strep-tag® also has additional benefits:
- Strep-Tactin®XT has a pM affinity for Twin-Strep-tag® and therefore, the combination is ideally suited for analysis of interactions with high affinity and slow dissociation rates.
- The reversibility of the interaction allows reuse of the sensor chips
- Efficient and easy one-step purification method - no tedious additional biotinylation
As a result, using the Twin-Strep-tag®/Strep-Tactin®XT system is cost-efficient and time-saving and the preferred choice when planning kinetic measurements such as SPR.
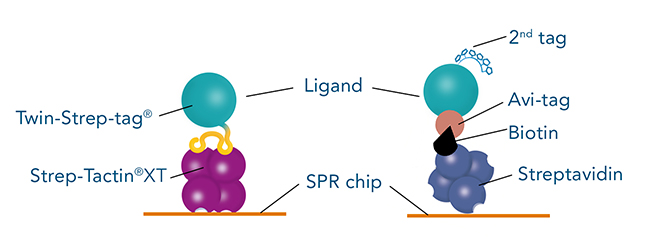
Twin-Strep-tag® – direct and time-saving protein purification with high yields
The ease of use, the high purity and yield are all features that indicate that Twin-Strep-tag® is superior to Avi-tag for protein purification. On top of that, the high affinity to Strep-Tactin®XT offers the possibility for efficient ligand immobilization necessary for SPR or other analyses of binding kinetics, making the Avi-tag essentially redundant.
Twin-Strep-tag® provides pM affinity to study high affinity interactions
SPR analysis requires immobilization of a ligand on a biosensor chip. To achieve this, the Avi-tag/streptavidin interaction is commonly used, because the binding is characterized by an exceptionally high affinity. Another interaction that offers the possibility for efficient immobilization is the binding of Twin-Strep-tag® to Strep-Tactin®XT. With an affinity in a low picomolar range it can achieve similar results in SPR analyses as the Avi-tag/streptavidin combination. The half-life of Twin-Strep-tag®/Strep-Tactin®XT complex is 13 hours, enabling measurements of high affinity interactions over a long period of time.
The versatility of the Twin-Strep-tag®/Strep-Tactin®XT combination during purification and analyses makes it a great alternative to using the Avi-tag/streptavidin interaction. By choosing the Twin-Strep-tag®, the whole workflow will become a more time-saving and cost-effective process.
Reduce costs with regenerative Strep-Tactin®XT biosensor chips
An interaction analysis always includes several measurements. Therefore, the option of reusing a biosensor chip would be highly beneficial since it reduces the costs associated with an experiment.
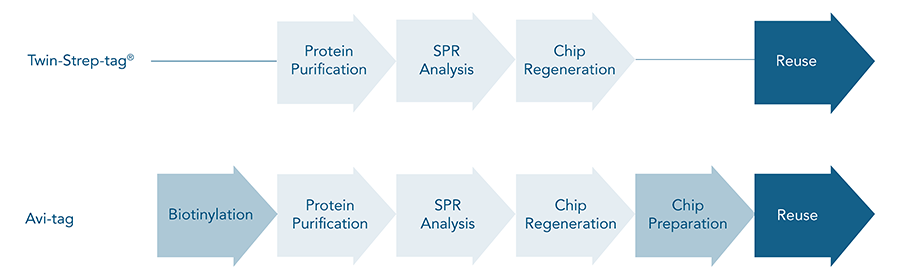
The possibility for biosensor chip regeneration exists for both the Avi-tag/streptavidin and the Twin-Strep-tag®/Strep-Tactin®XT system. However, important differences between these two systems are effectiveness, convenience of the procedure and costs for regenerating a chip.
Due to its high affinity, the Avi-tag/streptavidin interaction is too strong to dissolve and therefore the only efficient way to reuse a chip is to remove the whole ligand/streptavidin complex.
A new generation of streptavidin biosensor chips come with an established regeneration protocol. In this protocol, streptavidin is released during regeneration and must be re-applied before the next ligand application. A disadvantage of these streptavidin biosensor chips is that they are twice as expensive as Strep-Tactin®XT biosensor chips. A cheaper, but complex and often inefficient way is to use conventional streptavidin biosensor chips. For the regeneration of these chips, optimal regeneration conditions must be determined for each different ligand. After all, a new chip may be needed for each measurement anyway, since the procedure might also affect the ligand's activity.
In contrast to Avi-tag/streptavidin, which is a nearly irreversible binding, the Twin-Strep-tag®/Strep-Tactin® interaction can be dissolved easily. Although the binding affinity is very high, the addition of guanidine hydrochloride (GuHCl) causes the complete release of the strep-tagged ligand from Strep-Tactin®XT. Strep-Tactin®XT remains on the biosensor chip and is unaffected by this procedure. The protocol is fast and simple and allows regenerating the chips more than 30 times without adverse effects on functionality.
Stable performance of Strep-Tactin®XT biosensor chips after several regeneration cycle
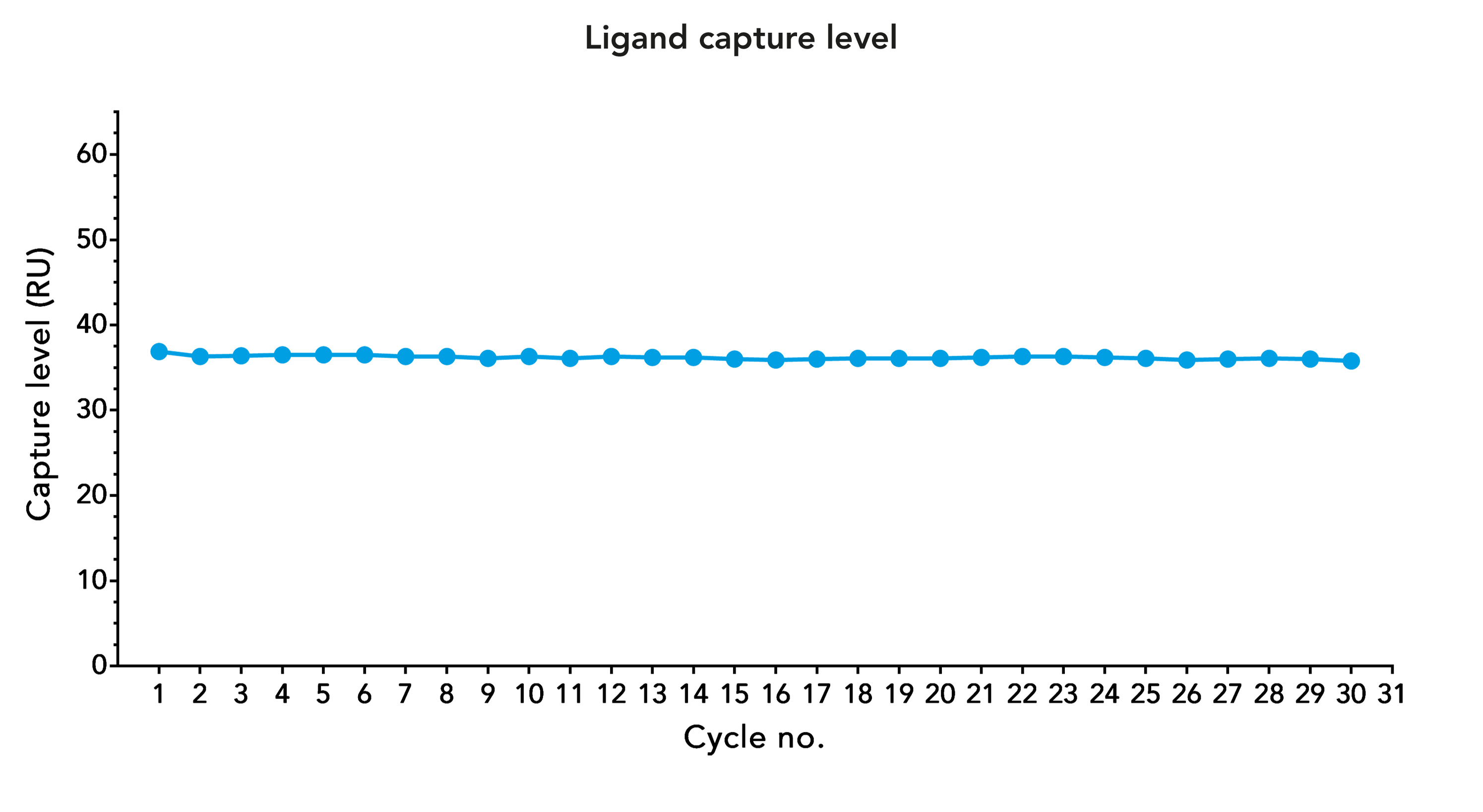
Fig. 4: Ligand capture level of CD45-Twin-Strep-tag® on a Strep-Tactin®XT coated CM5 chip after several regeneration cycles. Regeneration was performed with three consecutive 1-minute injections of 3 M GuHCl.
A superior alternative to His-tag/NTA - Stable, reversible and highly specific immobilization on SPR sensor chips using Strep-Tactin®XT
Xantec bioanalytics teamed up with IBA Lifesciences to introduce Strep-Tactin®XT/Twin-Strep-tag® system as a new derivative on SPR sensor chips.
The Strep-Tactin®XT/Twin-Strep-tag® system offers several key benefits over the traditional His-tag/NTA system for SPR sensor chips, including high affinity and binding stability in the low picomolar range, making it exceptionally reliable for protein immobilization. The regeneration process is straightforward, supporting over 100 cycles with minimal capacity loss, which enhances the system's usability and efficiency.
Additionally, the Strep-Tactin®XT/Twin-Strep-tag® system demonstrates significantly reduced nonspecific interactions compared to the His-tag/NTA system, particularly benefiting applications involving larger analytes. Immobilization is site-specific and occurs under physiological conditions, preserving high biological activity and ensuring reproducibility.
The Strep-Tactin®XT/Twin-Strep-tag® sensor chip variants are optimized for different applications such as protein-protein interactions, fragment screening, and concentration analyses. Overall, the system provides a more stable, specific, and efficient method for SPR sensor chip immobilization, addressing the limitations of the His-tag/NTA system and enhancing the reliability of SPR-based analyses.
Application examples
Strep-Tactin®XT
Strep-Tactin®XT binds with high affinity Twin-Strep-tag® proteins, making it perfectly suitable for SPR analysis. The examples show the coupling of Strep-Tactin®XT on different SPR chips and possible regeneration conditions for re-use of Strep-Tactin®XT-coated chips.
Coupling on CM5

We recommend to use the following conditions for optimal Strep-Tactin®XT coupling:
- 20-50 µg/ml Strep-Tactin®XT
- max. 20 mM acetate, pH 4.5
Using these conditions for Strep-Tactin®XT coupling, efficient and reproducible coupling results can be obtained.
Coupling on CM4

The membrane protein CB2 was fused with Twin-Strep-tag® and then immobilized on a CM4 sensor chip coated with Strep-Tactin®XT.
Used conditions:
- 50 mM Tris pH 7.5, 100 mM NaCl
- 10 % glycerol, 0.1 % DDM, 0.5 % CHAPS, 0.1 % CHS
Data was published in A. Yeliseev et al., Protein Expression and Purification 131 (2017) 109-118
Regeneration

Three different regeneration conditions were tested on Strep-Tactin®XT CM5 sensorchips:
- 10 mM NaOH / 500 mM NaCl
- 3 M MgCl2
- 3 M GuHCl
for three different Twin-Strep-tag® fusion proteins:
- mCherry-Twin-Strep-tag
- GAPDH-Twin-Strep-tag
- mAb-Twin-Strep-tag
We recommend the application of 3 M GuHCl for optimal regeneration conditions, since this buffer led to the best regeneration results for all test proteins. However, depending on the target protein, other buffers are applicable as well.
StrepMAB-Immo
Due to the nearly irreversible binding of Strep-tag®II proteins, StrepMAB-Immo can be used for SPR analysis. The example shows the stable capture of a Strep-tag®II fusion protein on a SPR chip coated with StrepMAB-Immo.

Chips for SPR were coated either with StrepMAB-Immo or with a competitive antibody (Competitor Q). Subsequently, a Strep-tag®II fusion protein was captured, and the binding stability was determined using a Biacore 3000.
During the washing phase, the recombinant Strep-tag® protein of interest remains tightly bound to StrepMAB-Immo, while a significant amount of Strep-tag® protein is washed off using the competitive antibody.

