Immunohistochemistry and immunocytochemistry
Amongst the immunostaining methods, immunohistochemistry is the most prominent one, in which whole tissues can be visualized, and thus, specific targets can be detected. In contrast to immunohistochemistry, in immunocytochemistry the focus is not on tissues but on cells. The advantage of both techniques is the possibility of imaging proteins in their proper histological context, which enables the localization of cellular compartments and can be detected using fluorescence microscopy. In both methods the sample fixation is essential to preserve the morphology and structure.
While both, chromogenic or fluorescent labels are utilized for detection, fluorescent labeling is especially popular due to the possibility of multicolor staining of different targets. StrepMAB-Classic or StrepMAB-Immo conjugated to different dyes can be applied for direct labeling of Strep-tagged targets. As viable alternative to antibodies, Strep-Tactin® and Strep-Tactin® XT with different fluorescent conjugates can be used as well. Due to their strong and highly specific interaction with Strep-tagged targets, both generate signals with high sensitivity, avoiding the need for a secondary antibody. However, when only small amounts of Strep-tagged protein are present, the unconjugated murine antibodies StrepMAB-Classic and StrepMAB-Immo can be used combined with a secondary antibody of choice.
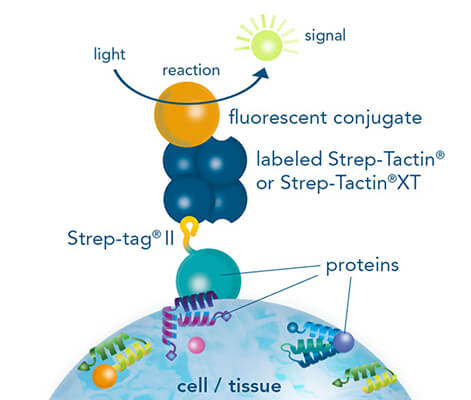
Immunohistochemistry/-cytochemistry using fluorescently labeled Strep-Tactin®
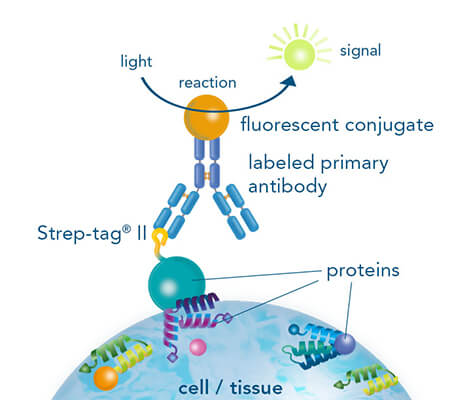
Application example
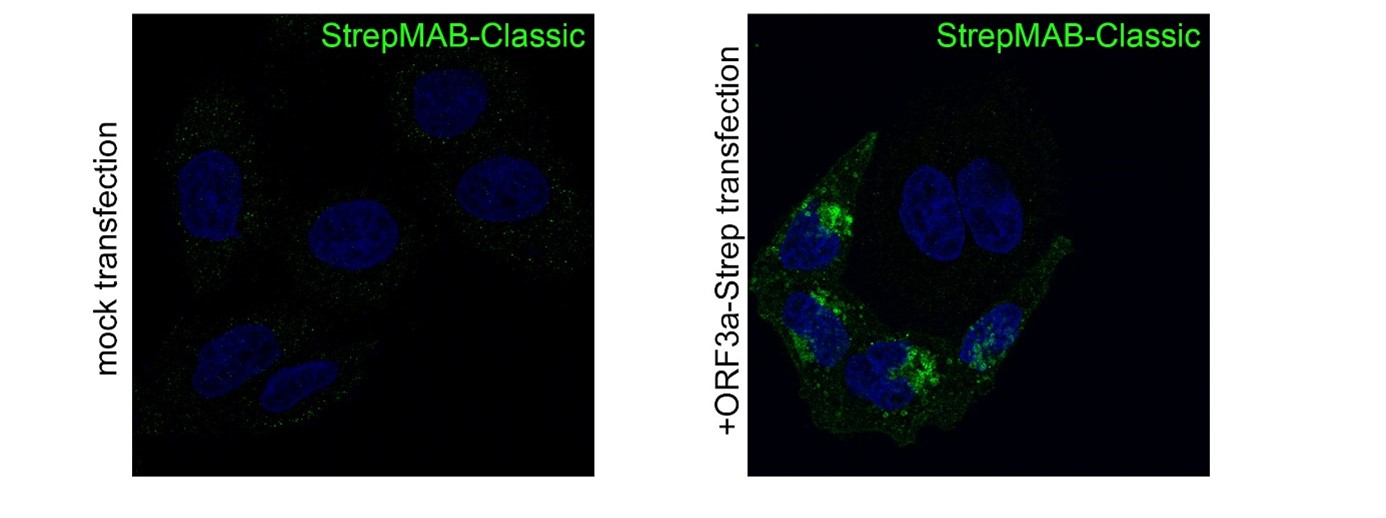
StrepMAB-Classic
Application: Confocal microscopy (Immunofluorescence)
Dilution: 1:500
HeLa cells were grown on glass coverslips, fixed (4% PFA/PBS), quenched (15 mM glycine/PBS), permeabilized (0.1% saponin/PBS), blocked (1% BSA, 0.01% saponin in PBS), and stained with StrepMAB-Classic (1:500) to confirm the expression of SARS-CoV-2 ORF3a-Twin-Strep-tag. Anti-mouse 488 was used as secondary antibody. Nuclei were stained with DAPI and the imaging occurred with a LSM700 confocal microscope (63×/1.4 NA oil immersion objective; ZEISS). Source: Data kindly provided from James Edgar, Department of Pathology at the University of Cambridge.

