Fluorescent cell staining for flow cytometry with Strep-Tactin® and Strep-Tactin®XT conjugates
The Strep-tag® technology provides different options for cell staining.
Direct vs. indirect staining
Direct staining of cells expressing a protein with a Strep-tag®II or a Twin-Strep-tag® on the surface
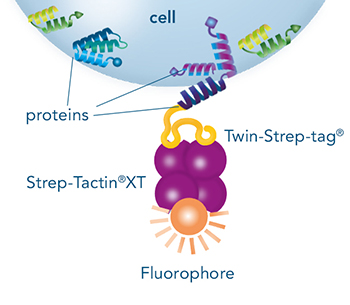
Indirect staining via a cell-binding protein (e.g. a Fab fragment, a MHC molecule or an antigen) that is fused to a Strep-tag®II or a Twin-Strep-tag®
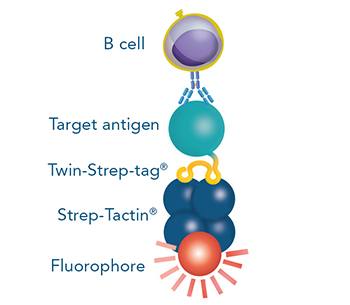
Direct staining
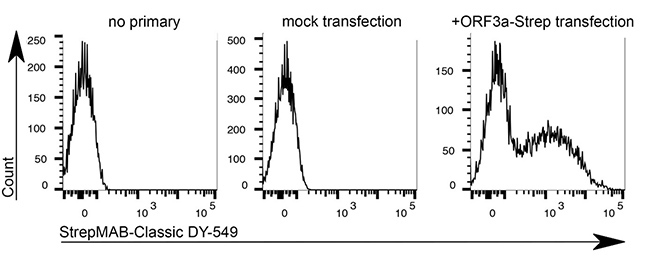
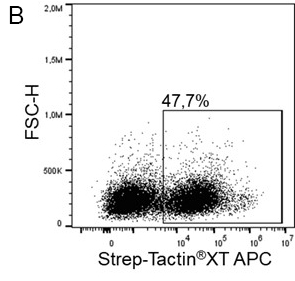
The Strep-tag®II or Twin-Strep-tag® are short peptide sequences that can be expressed on a cell surface without affecting the properties of the cell. Fusing one of the tags to a surface protein of choice enables easy selection of cells that successfully express this protein. MEXi cells expressing a surface protein fused to a Twin-Strep-tag® were mixed with native MEXi cells at a ratio of approximately 1:1. 1 x 106 total cells were stained with Strep-Tactin®XT DY-649 at a dilution of 1:5000* (A) or with Strep-Tactin®XT APC at a dilution of 1:1000* (B). Staining was performed in 100 µl Buffer CI (1x PBS with 1 mM EDTA and 0,5% BSA) for 30 min at 4°C.
*Titration of optimal dilutions might be required for individual experimental setups. In addition, the choice of conjugate depends on factors such as protein density on the cell surface. Due to their brighter staining, PE and APC conjugates can be advantageous for low expressed targets.
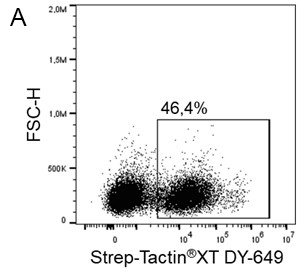
Indirect staining via strep-tagged protein (Fab)
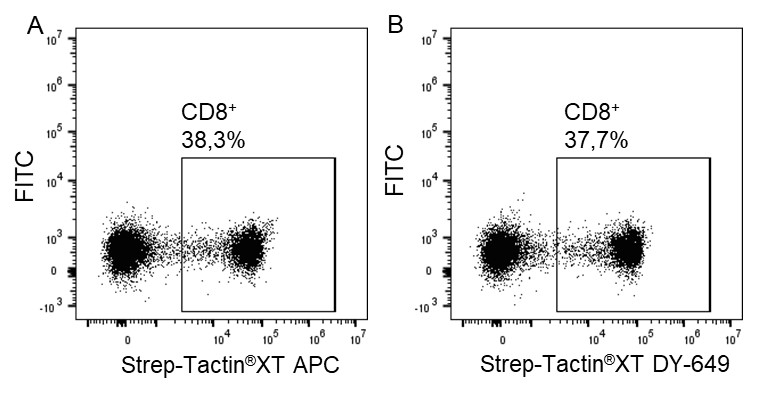
For cytotoxic T cell staining, 200 ng anti-human CD8 Fab fragment (50 kDa) fused to a Twin-Strep-tag® was pre-incubated with 75 ng of Strep-Tactin®XT APC (A) or Strep-Tactin®XT DY-649 (B) for 10 min at 4 °C. The formed complexes were added to 5 x 106 peripheral blood mononuclear cells (PBMCs) and incubated for 20 min at 4 °C in the dark. Afterwards, additional staining antibodies were added. Both fluorescent conjugates achieved clear separation of positive and negative cell populations, demonstrating a flexible choice of fluorophores for highly expressed target proteins such as CD8 on the surface of T cells.
Indirect staining of T cells via strep-tagged protein (MHC)
For staining low affinity targets such as T cell receptors, multimerization of the ligand is required to enable stable binding of the detection reagent to the cell. This multimerization can be achieved with Strep-Tactin® or Strep-Tactin®XT PE or APC conjugates.
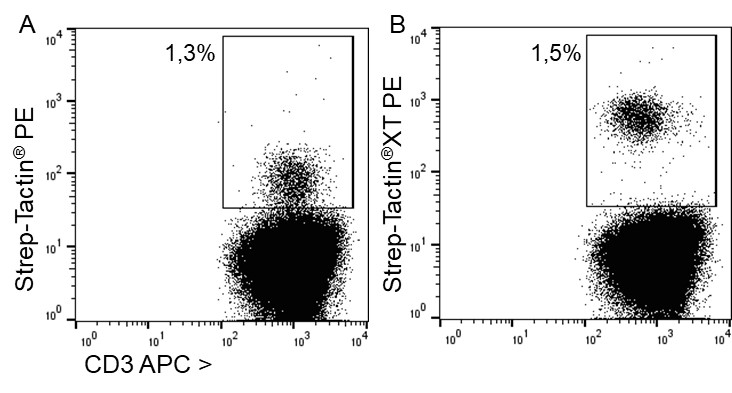
For antigen specific T cell staining, 200 ng of an MHC class I molecule fused to a Twin-Strep-tag® (50 kDa) refolded with a cytomegalovirus (CMV)-derived peptide was pre-incubated with 75 ng of Strep-Tactin® PE (A) or Strep-Tactin®XT PE (B) for 15 min at 4 °C. 1 x 107 PBMCs in 100 µl buffer were added to the staining complexes and incubated for 20 min at 4 °C. Afterwards, additional staining antibodies were added.
Strep-Tactin®XT PE achieved a brighter staining intensity than Strep-Tactin® PE, indicating that Strep-Tactin®XT PE and APC are the preferred conjugates for low affinity and low expressed targets such as specific T cell receptors. Strep-Tactin®XT DY-649, DY-549 and DY-488 conjugates are not recommended for this approach, since their lesser brightness does not achieve sufficient staining (data not shown).