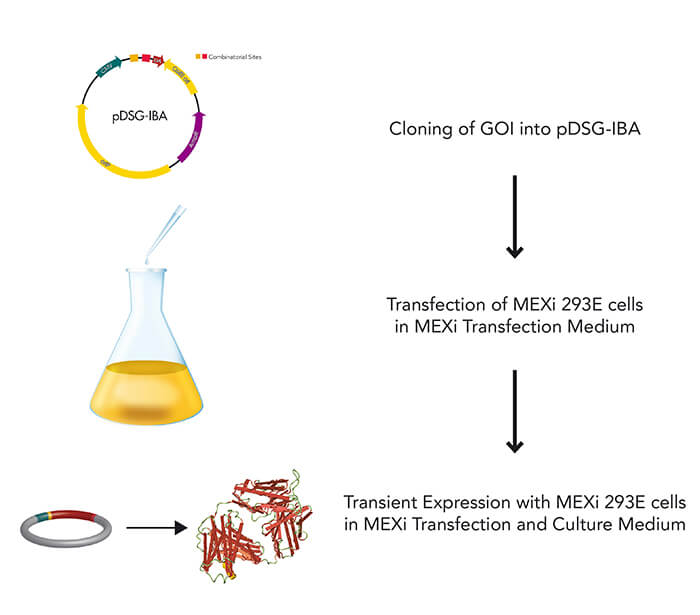
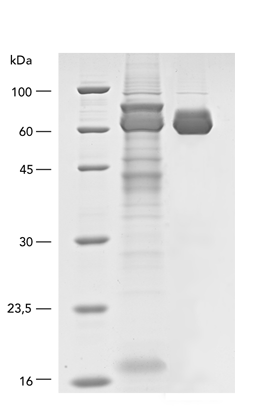
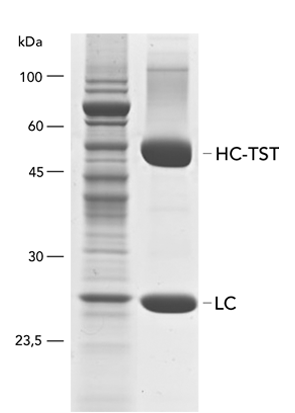
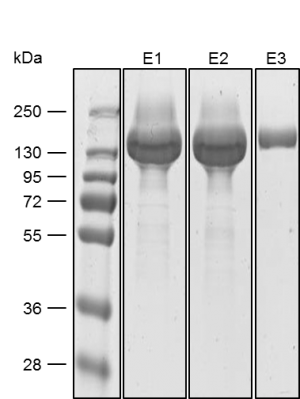
MEXi mammalian expression system
Mammalian cells are often used for the expression of recombinant proteins when complex glycolysation and accurate protein folding are important. The MEXi system provides an affordable mammalian protein expression system along with all essential components. MEXi-293E cells are human embryonic kidney (HEK) cells derived from the 293 cell line, adapted to suspension growth in an affordable cell culturing medium with a low biotin content. This allows highly efficient and specific purification of recombinant proteins via Strep-Tactin®XT under physiological conditions.
The MEXi system uses transient expression rather than stable transformation, to enable a quick and short-term production of the recombinant protein for several days after DNA transfection. This happens via episomal replication and expression of the GOI without the need for a prior integration by making use of the EBNA1 protein in combination with oriP-harboring plasmids. Further, the system deploys a polyethylenimine-based transfection, which is an affordable and quick method suitable even for hard-to-transfect cell lines, while simultaneously avoiding cytotoxic effects as in lipidbased techniques, or complicated viral packaging. Products beyond protein expression and purification are available from IBA Lifesciences, such as reagents for specific detection, using the Strep-tag® technology. This allows the entire research task to be easily performed using synchronized protocols within one technology platform.